How Many Types Of Distillation Are There

Hey there, curious minds and flavor adventurers! Ever found yourself sipping on a perfectly chilled gin, marveling at its crisp botanical notes, or perhaps enjoying a rich, smooth whiskey that warms you from the inside out? Well, guess what? You've been experiencing the magic of distillation! And today, we're diving headfirst into the wonderfully varied world of how we get those fantastic spirits, not to mention a whole bunch of other cool stuff, separated and purified. It's like a culinary science experiment, but with way better results – and way less mess!
Now, you might be thinking, "Distillation? Isn't that just… heating stuff up and cooling it down?" And you'd be mostly right! At its core, that’s exactly what it is. It’s all about separating components of a liquid mixture based on their different boiling points. Think of it like this: water boils at 100°C (212°F), while the alcohol in your favorite drink boils at a much lower temperature, around 78.37°C (173.07°F). By carefully heating the mixture, we encourage the alcohol to vaporize first. Then, we capture and cool that vapor, turning it back into a liquid – a liquid with a much higher alcohol concentration. Voila! Pure, potent goodness.
But here's where it gets really exciting: it's not just a one-size-fits-all operation. Nope! The world of distillation is bursting with different methods, each designed to achieve a specific outcome. It’s like having a whole toolbox of techniques to create different flavors, different textures, and even different products altogether. Pretty neat, huh?
So, How Many Types of Distillation Are There? Let's Count 'Em!
While the scientific community might quibble over exact categorizations (they do love their definitions, don't they?), for us everyday enthusiasts, we can generally break down the most common and impactful types into a few key players. Let's explore these, shall we? Get ready to have your mind gently tickled with some fascinating processes!
The OG: Simple Distillation
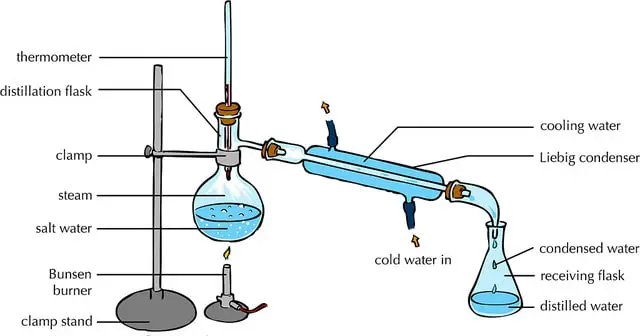
This is your foundational method, the granddaddy of them all. Think of it as the most straightforward way to get the job done. You heat a liquid, the vapor rises, it condenses, and you collect the purified liquid. Simple, right? It's fantastic for separating liquids with significantly different boiling points, like getting pure water from salt water (no more salty tea, thank goodness!). For spirits, it's often the first step in creating many base alcohols, laying the groundwork for more complex creations.
Imagine you’re making a homemade essential oil. You might put plant matter in water, heat it up, and the steam carries the fragrant oils. That steam then cools down, and you collect those precious oil droplets. Simple distillation is the workhorse behind many of these aromatic treasures. It’s efficient, it’s effective, and it’s the bedrock of so much that we enjoy.

The Speedy Gonzales: Fractional Distillation
Now, things get a little more sophisticated. What if your liquids have pretty close boiling points? Like, say, the different alcohols and flavor compounds in a fermented mash? That’s where fractional distillation shines. This is the rockstar of spirit production, responsible for that beautiful range of flavors you find in whiskey, vodka, rum, and gin.
The key difference here is the addition of a fractionating column. This is essentially a tall column placed between the boiling flask and the condenser. Inside, there are usually plates, packing material, or other surfaces that create a large surface area. As the vapor rises through the column, it cools and condenses on these surfaces. This condensed liquid then gets reheated by the rising vapor, causing further vaporization and condensation. With each "cycle" or "plate" up the column, the vapor becomes progressively richer in the more volatile component (the alcohol, in our case).
Think of it like a series of mini-distillations happening all at once. It’s a much more precise way to separate components with similar boiling points. This process allows distillers to control the character of the spirit by carefully managing how many "theoretical plates" the vapor passes through. More plates generally mean a purer, more neutral spirit (think vodka), while fewer plates allow more of the original fermented flavors to carry through (think some whiskies). It’s a dance of temperature and pressure, all to coax out the perfect flavor profile!

The Gentle Touch: Steam Distillation
This method is a favorite when dealing with heat-sensitive compounds, like many delicate essential oils from flowers or herbs. Instead of boiling the material directly, steam distillation uses steam to gently vaporize the desired components. The steam passes through the plant material, picking up the volatile oils without scorching or degrading them.
Imagine making rosewater. You wouldn't boil the rose petals directly; you’d be left with sad, cooked petals! Instead, you’d pass steam through them. The steam gently coaxes out the beautiful rose fragrance. This vapor, now infused with the essence of the rose, is then condensed and collected. It’s a much kinder, gentler approach, preserving the most ethereal aromas. This is how we get those incredibly pure and fragrant essential oils that we use in perfumes, aromatherapy, and even some culinary applications.
The Vacuum Cleaner of Purity: Vacuum Distillation
Ever wondered how they get those super-refined oils or certain pharmaceuticals? They often use vacuum distillation. Remember how alcohol boils at a lower temperature under normal pressure? Well, by reducing the pressure around the liquid (creating a vacuum), you can significantly lower the boiling point of almost any substance. This is a game-changer for compounds that would degrade or decompose at their normal boiling temperatures.
Think of it as giving your liquid a vacation to a low-pressure environment. It can relax and boil at a much cooler, safer temperature. This is absolutely crucial for certain delicate organic compounds and for achieving extremely high levels of purity without damaging the product. It's a bit more technical, requiring specialized equipment, but the results are often spectacular in terms of product integrity and purity.
The One-Stop Shop: Azeotropic Distillation (and Other Tricks!)
Now, this one is a bit more niche but incredibly important for specific applications, especially in chemistry. Sometimes, you have a mixture that forms an azeotrope. This is a mixture of liquids that has a constant boiling point, meaning it boils and vaporizes without changing its composition. It’s like they’re stuck together, refusing to separate by simple boiling!
To break this stubborn partnership, chemists use tricks like adding a third component (an entrainer) that forms a new, lower-boiling azeotrope, which can then be distilled off. Azeotropic distillation is key for making things like absolute ethanol (100% pure alcohol), which is essential for many scientific and industrial uses. It’s a clever workaround for a chemical puzzle!
![6 Types Of Distillation And Definition [Explained In Detail]](https://chemicaltweak.b-cdn.net/wp-content/uploads/2020/11/types-of-distillation-768x402.jpg)
And believe me, there are even more variations and specialized techniques out there! We've got things like short-path distillation for super-sensitive compounds, wiped-film distillation for highly viscous materials, and even molecular distillation, which operates under a very high vacuum to distill even large molecules. The world of distillation is vast and ever-evolving!
Why Does This Matter to You?
Well, beyond the fact that it’s fascinating science, understanding these different types of distillation helps you appreciate the craft behind your favorite beverages and products. It’s the reason why your single malt Scotch tastes so different from your vodka. It’s the reason why that lavender essential oil is so calming and pure. It’s a testament to human ingenuity and our desire to understand and manipulate the world around us.
So, next time you’re enjoying a perfectly crafted drink, take a moment to think about the journey it took to get there. Think about the precise temperatures, the clever columns, and the skilled hands that guided the process. It’s not just liquid; it’s a masterpiece of separation and purification, brought to you by the wonderful world of distillation.
And who knows? Maybe this little peek into the world of distillation has sparked a curiosity within you. Perhaps you're now inspired to learn more, to explore the science behind your favorite flavors, or even to experiment with your own little (safe!) projects. The journey of discovery is always open, and the world of distillation has so much more to reveal. So go forth, stay curious, and keep exploring the wonderfully separated wonders around you!
