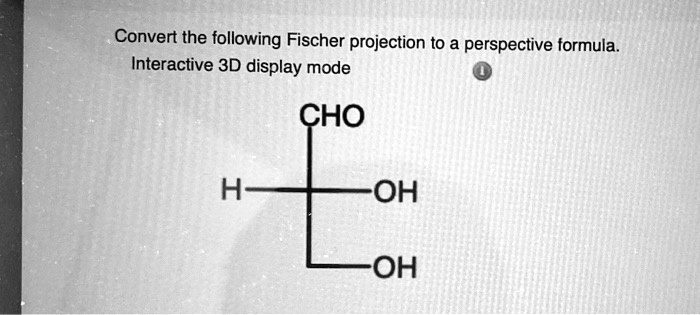
Convert The Following Fischer Projection To A Perspective Formula

Ever wondered if there's a secret code hidden within the squiggly lines and symbols that chemists use? Well, buckle up, because today we're diving into a bit of molecular magic that's surprisingly fun and incredibly useful: transforming a Fischer projection into a perspective formula. Think of it like taking a flat, almost cartoonish drawing of a molecule and bringing it to life in 3D, giving us a much clearer picture of its true shape and how it interacts with the world.
Why is this cool? Because molecules aren't just flat drawings; they exist in a three-dimensional space. Understanding their 3D structure is crucial for so many reasons, especially in fields like drug discovery, biochemistry, and even food science. The way a molecule folds and twists can determine if it tastes like a strawberry, binds to a specific protein in your body, or has a particular therapeutic effect. So, getting that 3D perspective right is like having x-ray vision for the molecular world!
From Flatland to 3D: The Transformation
You've probably seen a Fischer projection. It's that handy way of drawing a molecule where the longest carbon chain is represented vertically, and the horizontal lines stick out towards you. It's great for quickly showing stereochemistry, especially for molecules with multiple chiral centers like sugars or amino acids. However, it can sometimes be a bit ambiguous when you're trying to visualize the actual spatial arrangement of atoms around a chiral center. That's where the perspective formula comes in.
A perspective formula, also known as a wedge-and-dash formula or 3D representation, uses wedges to show bonds coming out of the page towards you, dashes to show bonds going back behind the page, and solid lines for bonds lying flat in the plane of the page. This gives us an immediate sense of depth and allows us to truly grasp the molecule's three-dimensional architecture. The real beauty of this conversion is that it helps us understand concepts like chirality and enantiomers much more intuitively.
Why Bother? The Perks of 3D Vision
So, beyond just looking cooler, what are the actual benefits of converting from a Fischer projection to a perspective formula? For starters, it significantly improves our ability to predict and understand a molecule's reactivity. The spatial arrangement of atoms can influence how easily one molecule can approach and interact with another. Think of it like trying to fit two oddly shaped puzzle pieces together – their orientation matters!

Furthermore, this 3D view is absolutely essential for understanding biological interactions. Many biological processes rely on a precise fit between molecules, much like a lock and key. A drug molecule, for example, needs to have the correct 3D shape to bind to its target receptor in the body. If the drug has the wrong stereochemistry (think of it as being the left-handed version when the lock needs the right-handed key), it might not work at all, or worse, it could have unintended and harmful side effects.
This is precisely why the conversion from a Fischer projection to a perspective formula is a fundamental skill in organic chemistry and biochemistry. It bridges the gap between a simplified representation and the complex reality of molecular shapes.
SOLVED: Convert the following Fischer projection to a perspective
For students learning about stereochemistry, this visual translation is a game-changer. It helps demystify concepts like R/S configurations, diastereomers, and meso compounds. Being able to mentally (or physically!) rotate a molecule and see it from different angles in 3D is a powerful tool for problem-solving and deeper comprehension. It moves you from memorizing rules to truly seeing the molecular world.
Let's take a common example: a simple sugar like glucose. In its Fischer projection, it might look like a straightforward chain. But in its perspective formula, you can truly appreciate how the hydroxyl groups are arranged, which dictates its properties and how it's recognized by enzymes in our bodies. This 3D understanding is what allows scientists to design artificial sweeteners that mimic the taste of sugar or to develop enzymes that can break down complex carbohydrates.
The process itself, while it might seem daunting at first, is quite logical. It involves a set of rules and a bit of spatial reasoning. Once you get the hang of it, you'll find yourself instinctively visualizing molecules in 3D, which makes tackling more complex chemical structures and reactions a lot more manageable and, dare I say, enjoyable!